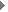
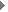
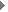
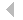
| IRG II – ENGINEERING MATERIALS and TECHNIQUES for BIOLOGICAL STUDIES at CELLULAR SCALES | |
| Coordinator: George M. Whitesides | |
| Donald E. Ingber (Harvard Med. School) Eric Mazur (DEAS, Physics) David R. Nelson (Physics, DEAS) David R. Reichman (Chemistry) Mara G. Prentiss (Physics) |
Aravintham Samuel (Physics) |
Collaborators: Howard Berg (Biology,
Physics) and N. Michelle Holbrook (Biology) |
|
This IRG focuses on the materials science for the study of biological systems at the scale of the cell. It has made considerable progress combining studies of imaging to study the structure and properties of cells and confining cells on controlled structures to investigate the response of the cell. In addition, new methodology was developed to locally disrupt structures within the cell using femtosecond laser light, and this was applied to the investigation of the behavior of neurons in an organism. Ingber has continued his studies focusing on development and applications
of new micromaterials and nanotechniques for analysis of the living
mammalian cells. Working in collaboration with Whitesides, he cultured
living cells on micropatterned substrates, self-assembled monolayers,
and flexible polydimethylsiloxane polymers and used these methods to
control the shape and position of cells, as well as regulate their
growth, differentiation, contractility, motility, and death. They also
developed and applied microfluidic techniques to place cells and molecules
in defined patterns and positions and to deliver soluble molecules
to precise locations and subcompartments within adherent cells. With
Mazur, he used femtosecond lasers to create a nanosurgical technique
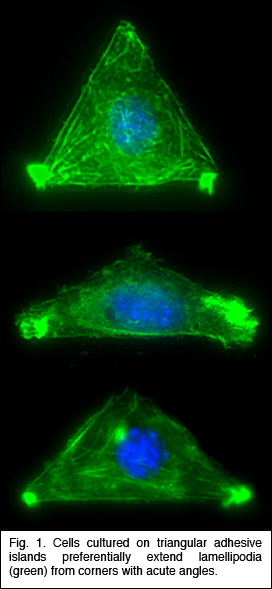
that The initial step in all cell motility is accomplished by the extension of actin-rich processes called lamellipodia. Ingber has shown that when single cells are cultured on individual cell-sized square adhesive islands created with microcontact printing and coated with fibronectin, these square cells preferentially extend lamellipodia from the corners relative to the sides when stimulated by motility factors. He now has used different shaped islands created with the same micropatterning technique to demonstrate that cells preferentially extend these motile processes from acute, rather than obtuse, angles (Fig. 1), and that they deposit new fibronectin fibrils in their corner regions. The physical determinants that he uncovered are critical for directional migration and may also be used as design criteria for creating engineered substrates that promote tissue regeneration. Mazur has developed a technique to disrupt submicrometer-sized organelles within living cells or tissue without affecting the surrounding material or compromising viability of the cell or organism. This method, called nanosurgery, received substantial media attention, and was featured in Nature Science Update and The Boston Globe (front page, Nov. 22, 2003). Working with a new member of the MRSEC, Asst. Prof. Samuel, he is studying a nematode worm Caenorhabditis elegans, whose behavior is encoded in the structure and function of its neural network, which comprises exactly 302 neurons that interconnect in the same way in every adult animal. Using tightly focused femtosecond infrared laser pulses, they precisely and reproducibly snip individual wires of an otherwise intact and invariant wiring diagram. By analyzing the behavior of the operated animals, they determine the computational effects of blocking information transmission at specific points. In a preliminary experiment, they severed
fibers Zhuang has begun to unravel
the molecular mechanisms underlying the nuclear trafficking of viral
genes. Many viruses deliver their genomes
to the cell nucleus for replication and expression. However, little
is known about how synthetic gene-delivery materials help targeting
foreign genes into the cell. Therefore, efforts to combat viral diseases
and to improve gene therapy could both benefit from new experimental
techniques for investigating the nuclear trafficking of genetic materials.
She has developed a physical technique to study gene trafficking by
tracking single genetic carriers in living cells using sensitive fluorescence
microscopy, and has applied this technique to study the nuclear trafficking
of influenza genes, packaged in the form of viral ribonucleoproteins
(vRNPs) (Fig. 3). Prentiss demonstrated that the unzipping of heterogeneous double stranded DNA proceeds by a series of jumps, as predicted earlier by Nelson. This experiment demonstrated that thermal fluctuations result in variations in the number of unzipped base pairs as a function of time, even for identical molecules under identical conditions. She also made the first measurement of the phase diagram for unzipping of dsDNA in the force temperature plane. Nelson’s theory correctly predicts the critical force at physiological temperatures, but departs strongly from the observations at temperatures outside of the physiological range. They believe that hairpin formation in the ssDNA and bubble formation in the dsDNA reduce the free energy difference between ssDNA and dsDNA at high temperatures, allowing the strands to unzip at lower forces than predicted. Weitz is studying the physical properties of networks of entangled and crosslinked actin, a semi-flexible polymer that is a major constituent of the cellular cytoskeleton. The mechanical properties of these semi-flexible polymer networks are determined by a competition of entropic and enthalpic effects and serve as an intermediate between classical networks of flexible polymers and rigid rods. Embedding colloidal particles in the F-actin networks and measuring their thermal motion probes the microstructure and mechanics of network. In collaboration with Reichman and an REU student, they probed spatial and temporal fluctuations in the network microstructure and proposed a framework in which to understand these in terms of the thermally driven fluctuations of single actin filaments. To further develop the applicability of microrheology, he collaborated with Whitesides to develop new coatings for the tracer particles used to probe biological samples. They developed a robust protocol for binding short poly(ethylene glycol) (PEG) chains to the surfaces of colloidal particles. These PEG-coated beads resist protein adsorption onto the colloid surface and enable precise characterization of bio-materials using microrheology techniques. |
|

Last Modified April 27, 2004.
 allows them to precisely disrupt molecular structures inside living
cells without altering surrounding structures or compromising cell
viability. With Weitz, he analyzed the biophysical basis of molecular
and organelle transport within the cytoplasm. With Prentiss, he applied
micromagnetic methods to guide cellular self-assembly into specific
tissue patterns, and used micro- and nano-magnetic approaches to probe
cellular mechanics and develop magnetically actuatable living cellular “switches.” Through
this combination of approaches from microelectronics, micromagnetics,
optics, biophysics and molecular cell biology, they are gaining greater
insight into the structural basis of cell regulation. These studies
will help establish a foundation of knowledge relating to cellular
microsystems that will form the basis of tomorrow’s microsensors,
biochips, and cell-based microdevices.
allows them to precisely disrupt molecular structures inside living
cells without altering surrounding structures or compromising cell
viability. With Weitz, he analyzed the biophysical basis of molecular
and organelle transport within the cytoplasm. With Prentiss, he applied
micromagnetic methods to guide cellular self-assembly into specific
tissue patterns, and used micro- and nano-magnetic approaches to probe
cellular mechanics and develop magnetically actuatable living cellular “switches.” Through
this combination of approaches from microelectronics, micromagnetics,
optics, biophysics and molecular cell biology, they are gaining greater
insight into the structural basis of cell regulation. These studies
will help establish a foundation of knowledge relating to cellular
microsystems that will form the basis of tomorrow’s microsensors,
biochips, and cell-based microdevices.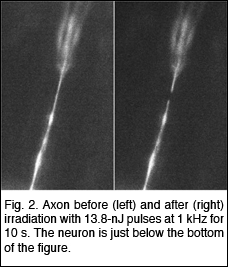 of the ASH neuron which plays a crucial role in the osmotic
avoidance behavior, nose
touch avoidance, and chemotaxis to certain compounds. Severing the
ASH neuron should block transmission of sensory information from the
sensory cilia to the synaptic connections of the ASH neuron and hence
all downstream neurons. Hence, by snipping two wires, belonging to
the left and right ASH neurons, they eliminate the behavior underpinned
by ASH. They have successfully snipped specific axons without affecting
the remainder of the worm (see Fig. 2), and are currently
evaluating the effect on behavior. Using laser nanosurgery, they will
be able
to assign functions to parts of neurons in the worm.
of the ASH neuron which plays a crucial role in the osmotic
avoidance behavior, nose
touch avoidance, and chemotaxis to certain compounds. Severing the
ASH neuron should block transmission of sensory information from the
sensory cilia to the synaptic connections of the ASH neuron and hence
all downstream neurons. Hence, by snipping two wires, belonging to
the left and right ASH neurons, they eliminate the behavior underpinned
by ASH. They have successfully snipped specific axons without affecting
the remainder of the worm (see Fig. 2), and are currently
evaluating the effect on behavior. Using laser nanosurgery, they will
be able
to assign functions to parts of neurons in the worm. Single-vRNP
trajectories show that vRNPs are transported in the cell by diffusion.
She has identified
two distinct types of
interactions between the vRNPs and the nuclear envelope. Her experiments
provide new insights into the regulation mechanisms for the nuclear
import of vRNPs; the influenza M1 protein down-regulates the nuclear
import of vRNP by inhibiting the binding of vRNPs to the nuclear envelope.
This single-gene tracking approach will find broad application in the
investigation of genetic trafficking in cells.
Single-vRNP
trajectories show that vRNPs are transported in the cell by diffusion.
She has identified
two distinct types of
interactions between the vRNPs and the nuclear envelope. Her experiments
provide new insights into the regulation mechanisms for the nuclear
import of vRNPs; the influenza M1 protein down-regulates the nuclear
import of vRNP by inhibiting the binding of vRNPs to the nuclear envelope.
This single-gene tracking approach will find broad application in the
investigation of genetic trafficking in cells.