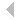
| SEED PROJECTS | |
| Jaques Dumais (OEB) Cynthia M. Friend (Chemistry, DEAS) Roy G. Gordon (Chemistry) L. Mahadevan (DEAS, OEB) Charles M. Marcus (Physics) |
Scot T. Martin (DEAS) |
Number of graduate students: 3 |
|
BASIS SETS for INTERFACE DESIGN Martin and Friend are
developing chemical tools for shaping interfaces on length scales ranging
from nanometers
ATOMIC LAYER DEPOSITION of VANADIUM OXIDE FILMS Gordon and Marcus discovered the first reaction known
for ALD of vanadium(III) oxide, V2O3. It uses vanadium(IV) chloride,
VCl4, vapor and water vapor, alternately supplied to a substrate surface
heated to around 300 C. They determined stoichiometry of the V2O3
film by Rutherford Backscattering Spectroscopy (RBS). Its X-ray diffraction
pattern agreed with the known phase of bulk V2O3 (Fig. 2) BIOMATERIALS and PHYSIOLOGY To take advantage of the arrival of several new faculty members at Harvard, and to further evolve the direction of the Harvard MRSEC towards materials science of biology, we established a new seed project. We expect that this seed project may well evolve into a full IRG, which will allow the direction and content of the Harvard MRSEC to evolve into new areas. The new seed IRG focuses on understanding the collective behavior of active material and biological systems at the single cell and multi-cellular level. Since the environment of life is the material world, it poses constraints on what is possible and proffers advantages to those who can exploit it. The goal of this IRG is to understand active non-equilibrium systems that respond to and modify the environment around them. Thus the systems to be investigated include both plant and animal systems that can remodel and adapt themselves to the material environment. IRG members will use experimental and theoretical tools to relate structure to function. The aim in studying these systems is to understand how to mimic them artificially and harness the exquisite molecular and genetic control present in natural biological systems. The IRG will investigate the mesoscopic theory of cell motility and tissue aggregation. We are beginning to understand how single molecules generate forces. However, since the single cell is the simplest viable entity we have to understand how to scale up from the single molecule to thousands of them. This involves thinking about collective phenomena in which only a few coarse-grained variables are important. From a materials perspective, many intriguing questions are raised by the transduction of chemical energy to mechanical work on a collective level, the role of the material environment in determining the actual motion, and the physico-chemical basis for the remodeling of the internal cytoskeleton in response to external cues. The IRG will also investigate the origin and control of spatio-temporal patterns in active biomaterial systems. Just as many modern materials are hierarchical structures optimized for function, so are there biological counterparts. While it is clear that genetic information s crucial in controlling complex programs in such situations as morphogenesis that leads to structures on a range of length scales, experiments also show that the patterns are often quite robust and seemingly independent of molecular details, at least in certain regimes. Important materials-related questions include the role of the environment in pattern formation (e.g., substrates, boundaries), the physico-chemical basis for the generation of length scales and the kinetics of evolution, and the coupling between genetic signaling pathways and the physical process of pattern formation. Organismal physiology and the material environment will also be a topic investigated by the IRG. Going up to the level of whole organisms, physical and material constraints provide the boundaries within which plants and animals must operate as they vie with each other to exploit their environment. Integrating cellular responses to understand the motion of whole organisms represents a challenge in both the biological and the material context. Indeed, many functional material designs may have much to learn from whole organism biology in questions associated with growth, transport, adhesion and locomotion. All plant cells are surrounded by a stiff extracellular matrix—the cell wall. The plant cell wall is a complex composite material whose major components are a dense network of cellulose microfibrils, cross-linking glycans, structural and regulatory proteins, and a pectin matrix. The specific organization of these molecular components confers to the cell wall exceptional properties such as a high mechanical anisotropy, potential for extensive plastic deformation, and a variable material stiffness that can be modulated by pH or enzymes. Thus, the plant cell wall is a material whose range of behavior exceeds by far those of classical engineering materials. Dumais, Zwieniecki and Mahadevan will collaborate to understand how specific structural features of the wall determine its material properties, to determine structure-property relationships for the cell wall of the giant unicellular alga Nitella. Water transport systems in plants can autonomously control hydraulic resistance of the pathway in response to ionic concentration of the fluid. While water passes through the system, it crosses specialized cellulose membranes impregnated with pectin-based hydrogels that show a swelling-deswelling behavior in response to ion concentration in the circulating fluid. The fast response time is achieved by minimizing diffusion distances within the hydrogel. Mahadevan will lead an effort with Dumais and Zwieniecki to characterize the biophysical properties of cellulose membranes and the dynamic changes of membrane porosity in response to fluid quality. They will use cryo-SEM and ESEM. They will also do experiments to fabrication microfluidic systems using soft lithography utilizing properties of hydrogel to controlfluxes. This will explore the analogy between the tree fluid-handling system and microfluidics fluid system. The nematode C. elegans is a well-established model organism for genetics and developmental biology, and an increasingly important model organism for neurobiology. But the worm’s potential as a powerful model organism for whole organism biomechanics and as an active (muscular) material system is untapped. Mahadevan and Samuel propose to develop the worm, a biomechanical system with extraordinary functionality, as a tractable experimental system for reverse materials engineering and behavioral studies. The worm moves in snake-like undulations that enable it to swim through fluids, reptate through granular or visco-elastic material, bore through solids, and crawl on solid surfaces. These motions are powered by rhythmic contraction and relaxation of 82 muscle cells that line its ventral and dorsal sides. The worm does not independently drive the activity of each muscle cell. Instead, the worm initiates spatial and temporal patterns of muscular activity that generate its stereotyped snakelike body movements. During forward movement, bending waves travel from nose to tail. But the worm is also capable of reorienting itself through sharp turns and reversals. By controlling the parameters of these movements in response to the material properties of its environment through mechanosensory and proprioceptive feedback, the worm selects and maintains an optimal gait defined by the wavelength, amplitude, and frequency of its undulations. Mahadevan and Samuel will explore the connection between a worm’s mechanical environment and its strategy for locomotion. This understanding will be used to determine other methods to create mechanical structures that can move in a similar fashion. This new IRG
will complement the work in IRG2, which focuses
on the development of tools and techniques at the scale of a single
cell, to study the mechanical
properties of biological systems at the cellular
level. Members of the new IRG include:
|
|
Last Modified January 7, 2005.
 to
microns, and is attempting to understand the underlying chemical mechanisms
to allow him to
combine fundamental shapes into complex structures. He
investigated heterogeneous nucleation of manganese oxide on carbonate
surfaces
to understand how substrate morphology affects the occurrence,
directionality, and spatial distribution of the heterogeneous nucleation
of manganese
oxide on carbonate minerals. Flat {10
to
microns, and is attempting to understand the underlying chemical mechanisms
to allow him to
combine fundamental shapes into complex structures. He
investigated heterogeneous nucleation of manganese oxide on carbonate
surfaces
to understand how substrate morphology affects the occurrence,
directionality, and spatial distribution of the heterogeneous nucleation
of manganese
oxide on carbonate minerals. Flat {10 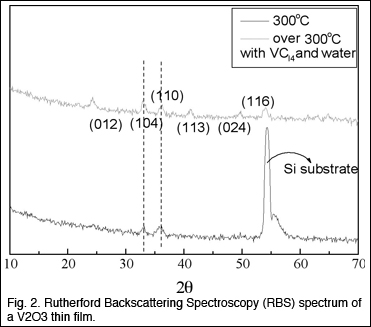 .
This reaction did not, however, produce the uniformly thick films
expected from an ALD process. The work of this seed has resulted in
the formation of a startup company,
.
This reaction did not, however, produce the uniformly thick films
expected from an ALD process. The work of this seed has resulted in
the formation of a startup company,