Filling a void in stem cell therapy
A new porous hydrogel could boost the success of stem-cell-based tissue regeneration
By Leah Burrows | September 14, 2015
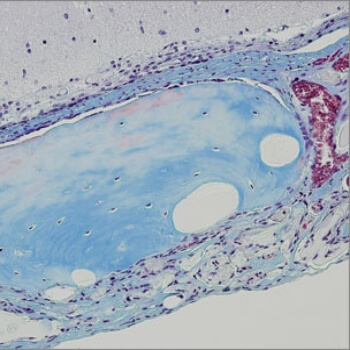
Stem cell therapies are often limited by low survival of transplanted stem cells and the lack of precise control over their differentiation into the terminal cell types needed to repair or replace injured tissues. Now, a team led by David Mooney, the Robert P. Pinkas Family Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), has developed a new strategy—embedding stem cells into porous, transplantable hydrogels—that has experimentally improved bone repair by boosting the survival rate of transplanted stem cells and influencing their cell differentiation.
Mooney, who is also a Core Faculty member at the Wyss Institute for Biologically Inspired Engineering at Harvard, and his team published their findings in the September 14 issue of Nature Materials. The team included Georg Duda, Ph.D., who is a Wyss Associate Faculty member and the director of the Julius Wolff Institute and Professor of Biomechanics and Musculoskeletal Regeneration at Charité – Universitätsmedizin Berlin, and Wyss Institute Founding Director Donald Ingber, M.D., Ph.D., who is also Professor of Bioengineering at SEAS and the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children's Hospital.
Stem cell therapies bear tremendous hopes for the repair of many tissues and bone or even the replacement of entire organs. Tissue–specific stem cells can now be generated in the laboratory. However, no matter how well they grow in the laboratory, stem cells must survive after they are transplanted and function correctly at the site of injury to be useful for clinical regenerative therapies. As of now, transplanted cell death remains a major challenge.
To improve the therapeutic ability of transplanted stem cells, Mooney's team has drawn inspiration from naturally occurring stem cell "niches". A 'stem cell niche' is a unique support system for stem cells consisting of other cell types and an extracellular molecular matrix that affects their fate.
Recently, Mooney's team as well as other researchers have identified specific chemical and physical cues from the niche that act in concert to promote stem cell survival, multiplication and maturation into tissue. Whereas chemical signals that control stem cell behavior are increasingly understood, much less is known about the mechanical properties of stem cell niches. Stem cells like those present in bone, cartilage or muscle cultured in laboratories, however, have been found to possess mechanosensitivities; meaning they require a physical substrate with defined elasticity and stiffness to proliferate and mature on.
"So far these physical influences had not been efficiently harnessed to propel real world regeneration processes," said Nathaniel Huebsch, a Graduate Student who worked with Mooney and who is the study's first author. "Based on our experience with mechanosensitive stem cells, we hypothesized that hydrogels could be leveraged to generate the right chemical and mechanical cues in a first model of bone regeneration."
Key to the method developed by Mooney's team is the combination of two water–filled hydrogels with very different properties. A more stable, longer lasting 'bulk gel' is filled with small bubbles of a second, so–called 'porogen' that degrades at a much faster rate, leaving behind porous cavities.
By coupling the bulk gel with a small peptide derived from the extracellular environment of genuine stem cell niches, and mixing it with a tissue–specific stem cell type as well as the porogen, the team can create a bone–forming artificial niche. While the bulk gel provides just the right amount of elasticity plus a relevant chemical signal to coax stem cells into proliferation and send them on their maturation path, the porogen gradually breaks down, leaving open spaces for the stem cells to expand into before they naturally migrate out of the gel structure altogether to form actual mineralized bone tissue.
In small animal experiments conducted so far, the researchers show that a void–forming hydrogel with the correct chemical and elastic properties provides better bone regeneration than transplanting stem cells alone. Of further interest, the maturing stem cells deployed by the hydrogel also induce nearby native stem cells to contribute to bone repair, thus further amplifying their regenerative effects.
"This study provides the first demonstration that the physical properties of a biomaterial can not only help deliver stem cells but also tune their behavior in vivo," said Mooney. "While so far we have focused on orchestrating bone formation, we believe that our hydrogel concept can be broadly applied to other regenerative processes as well."
The collaborative, cross–disciplinary work was supported by the Harvard University Materials Research Science and Engineering Center (MRSEC), which is funded by the National Science Foundation (NSF).
"This is an exquisite demonstration of MRSEC programs' high impact," said Dan Finotello, program director at the NSF. "MRSECs bring together several researchers of varied experience and complementary expertise who are then able to advance science at a considerably faster rate."
Additional funding was provided by: the National Institutes of Health; the Belgian American Education Foundation; the Einstein Foundation Berlin; the Berlin-Brandenburg School for Regenerative Therapies; the Harvard College Research Program; and NSF Graduate Research, Einstein Visiting, Harvard College PRISE, Herchel-Smith and Pechet Family Fund Fellowships.
