Hydrolysis Embrittles Poly(lactic acid)
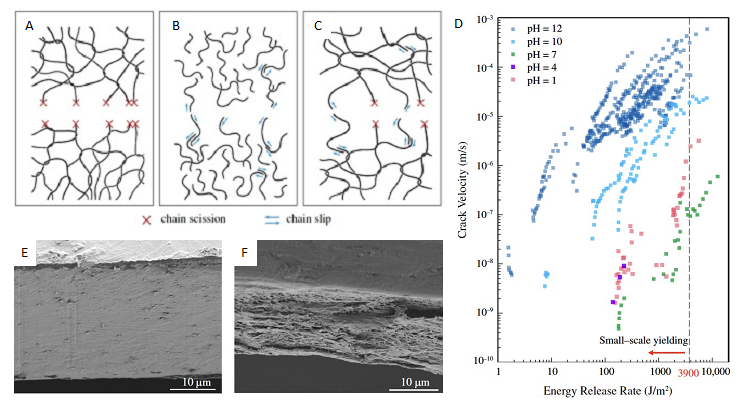
(A-C) Schematics of fracture by chain scission without chain slip, by chain slip without chain scission, and with chain slip and chain scission, respectively.
(D) Crack velocity as a function of energy release rate and pH.
(E-F) SEM images of brittle (pH 12) and ductile (pH 7) fracture surfaces, respectively.
Many biodegradable polymers contain chemical bonds that are susceptible to hydrolysis. A team at the Harvard MRSEC led by Suo and Vlassak showed that even a small load exposes the crack tip to water molecules from the environment, hydrolyzing ester bonds along the backbone of poly(lactic acid) and driving chain scission. When submerged in water under load bearing conditions, cracks in this polymer exhibit velocities that vary by orders of magnitude during on the pH and energy release rate. At high energy release rates, cracks grow fast and extensive plastic deformation is extensive leading to rough fracture surfaces. By contrast, at lower release rates, cracks grow slowly, plastic deformation is negligible, and the fracture surface is flat. This behavior must be considered when designing biodegradable polymers for future use.
Publication:
Shi, M., Q. Jiao, T. Yin, J.J. Vlassak, Z. Suo, "Hydrolysis embrittles poly(lactic acid)," MRS Bulletin 48, 45-55 (2023) ![]()
![]()
Zhigang Suo (Material Science & Mechanical Engineering) and Joost J. Vlassak (Material Science & Mechanical Engineering)
2022-2023 Harvard MRSEC (DMR-2011754)
