Entropy Favors Asymmetry in Colloidal Self-Assembly
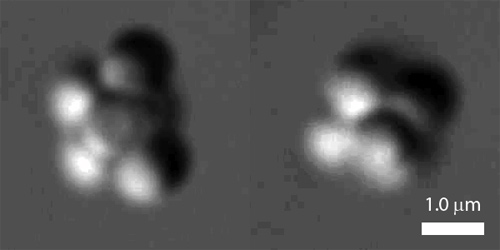
Two self-assembled colloidal clusters, as seen under the optical microscope. The cluster on the left, a tri-tetrahedron, and the cluster on the right, an octahedron, have the same energy. But in an experiment where both clusters are allowed to form randomly in solution, the less symmetric tri-tetrahedron occurs more than twenty times as often as the highly symmetric octahedron because of the many more ways to form the tri-tetrahedron. The findings illustrate, in a tangible way, what entropy is and how it determines the structures of small self-assembled materials.
David A. Weitz (Physics & Applied Physics)
Cynthia M. Friend (SEAS)
Guangnan Meng,
Natalie Arkus,
Michael P. Brenner (Applied Math), and
Vinothan N. Manoharan (Applied Physics, Materials, & Mechanical Engineering)
Harvard MRSEC (DMR-1420570)
